Copper Oxide and Sulfuric Acid Formula
Dissolving the solidified. Stannic acid refers to hydrated tin IV oxide SnO 2 which is.

Pdf Copper Dissolution In Concentrated Sulfuric Acid
SnO 2 6 HI H 2 SnI 6 2 H 2 O.

. Similarly SnO 2 dissolves in sulfuric acid to give the sulfate. SnO 2 dissolves in strong bases to give stannates with the nominal formula Na 2 SnO 3. Buy Nitric Acid Products Online Here Or By Phone.
Concentrated nitric acid 68 - 70 is a transparent colorless or yellowish fuming suffocating hygroscopic corrosive liquidThis chemical attacks almost all metals. SnO 2 2 H 2 SO 4 SnSO 4 2 2 H 2 O.

Solved Which Of The Following Shows The Balanced Equation Chegg Com
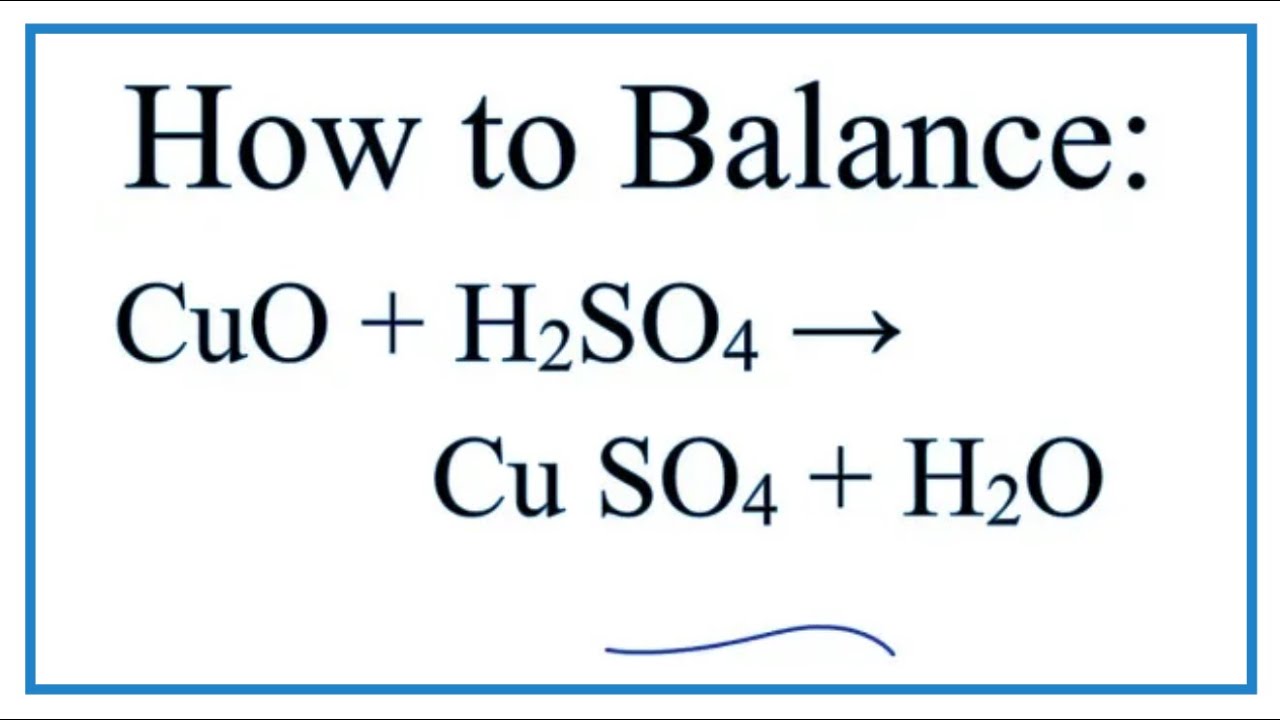
How To Balance Cuo H2so4 Cuso4 H2o Youtube

How To Write The Net Ionic Equation For Cuo H2so4 Cuso4 H2o Youtube

How To Write The Net Ionic Equation For Cuo H2so4 Cuso4 H2o Youtube
No comments for "Copper Oxide and Sulfuric Acid Formula"
Post a Comment